Crystalline Solids
Most solids have a crystalline structure of one kind or another. Crystalline solids are said to have both short range and long range order. A crystalline solid has short range order because the arrangement of the atoms within the crystalline structure, over short distances, is predictable. In a sodium chloride (common salt) crystal, for example, an equal number of sodium and chloride ions are arranged in a regular pattern. Each sodium ion is surrounded by six chloride ions, and each chloride ion is surrounded by six sodium ions. Because this pattern is repeated throughout the crystal, it also has long range order.
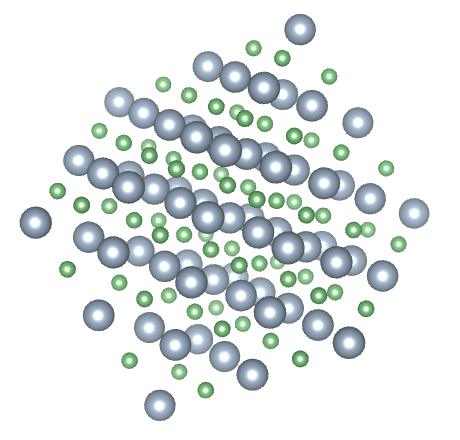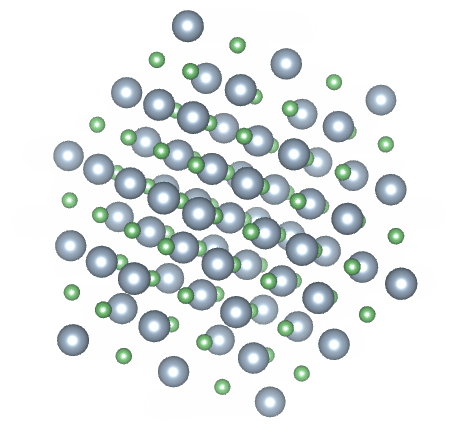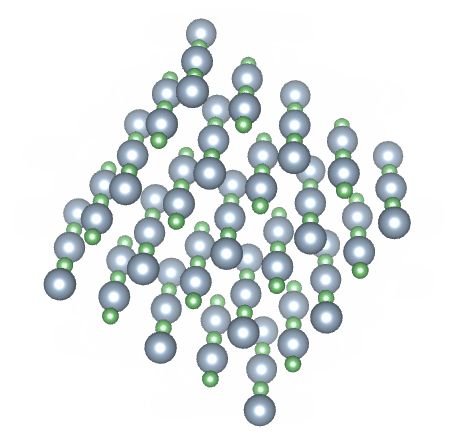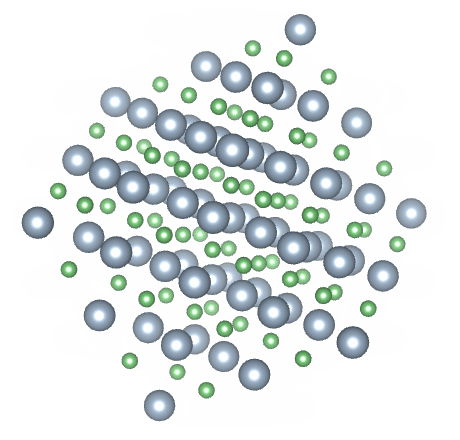
The crystalline structure of sodium chloride (NaCl)
The animation above was created using VESTA (Visualization for Electronic and STructural Analysis), a software package developed by Koichi Momma and Fujio Izumi that is distributed free of charge for academic, scientific, educational, and non-commercial use.
All crystalline solids have certain common characteristics. For example, the particles from which a crystalline solid is constructed (atoms, ions or molecules) are packed together fairly tightly. As a result, it is not easy to compress a crystalline solid. Having said that, the same substance can have a number of different crystalline structures, depending on the environment in which it is found and how it was formed. Ice is a good example, with fifteen different structural forms known to exist.
The uniformity of the internal structure of a crystalline solid means that the chemical bonds between the constituent particles all have the same strength. This results in the melting point of a crystalline solid having a very specific value, because when heat is applied, all of the bonds will break at the same temperature.
All crystalline solids demonstrate anisotropic behaviour. This means that the values of properties such as electrical conductance (how well the material conducts electricity), refractive index (how much light is bent as it passes through the material), and tensile strength (how much force is required to break a sample of the material apart) have different properties in different directions. They also demonstrate a property called cleavage, which means that when broken or cut, the pieces will have clean surfaces and/or straight edges.
Crystalline solids are generally classified according to the nature of the particles (atoms, ions or molecules) from which they are made, and the chemical bonds that hold the particles together. The four main categories are:
- Network covalent solids
- Ionic solids
- Metallic solids
- Molecular solids
