Introduction
Overview
The universe - as far as we know at the present time - is made up of energy and matter. Matter has mass, and occupies space. All physical objects, materials and substances are composed of matter in one form or another. You are no doubt familiar with the three most common states (sometimes called phases) in which matter can be found - solids, liquids and gases. We will also be looking at a fourth state of matter - also relatively common - called plasma.
In addition to these four states of matter, there are a number of more "exotic" states - some of which have been proven to exist, and others that are as yet only theoretical. These exotic states of matter occur (or in some cases are thought to occur) under extreme circumstances. Matter can behave in unexpected ways, for example, under extreme pressure, or at extreme temperatures. For the purposes of this discussion, we will confine ourselves to the four common states of matter.
It is thought that only about thirty percent of the universe consists of matter, and that the other seventy percent is energy. What's even more surprising is that we can only actually see about twenty percent of the matter in the universe. The rest of it is what scientists call dark matter, the exact nature of which is still somewhat of a mystery - but that's a story to be explored elsewhere.
The atom
Matter is composed of incredibly small building blocks called atoms. The atom is not the smallest kind of particle known to exist, however. Atoms are made up of even smaller building blocks which we collectively call subatomic particles. The three most important subatomic particles are called protons, neutrons and electrons. The protons and neutrons, which have approximately the same mass, form the core (or nucleus) of the atom. The electrons, which are much smaller than either neutrons or protons, orbit the nucleus at very high speeds.
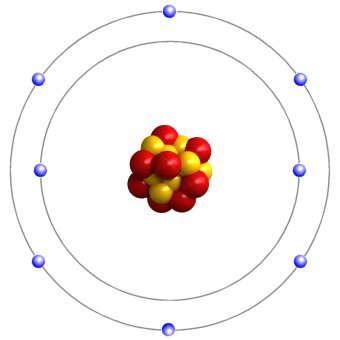
In an oxygen atom, eight electrons surround a nucleus containing eight protons and eight neutrons
It has been estimated that our planet contains approximately 1.33 × 10 50 atoms - an almost unimaginably large number! The atom is important because it is the smallest particle that can be identified as belonging to a particular chemical element, whereas the subatomic particles from which it is made up can be found in all chemical elements (with one or two notable exceptions).
The kind of atom we are dealing with will depend on exactly how many protons, neutrons and electrons the atom contains. This will determine to which chemical element it belongs. A chemical element consists of atoms of one particular kind only. So far, scientists have identified about one hundred and eighteen different elements. These elements are listed in the periodic table of elements.
Chemical bonding
Some of the elements discovered by scientists exist as single atoms. These elements are known as the inert gases or noble gases, because they do not react with other elements. Atoms of these elements do not combine with each other, or with the atoms of other elements. The reason for this is that, unlike most elements, the noble gases have a stable electronic configuration (we will talk more about what exactly that means elsewhere).
The atoms of most elements, however, rarely exist in nature in splendid isolation. They tend to get together with other atoms in a process known as chemical bonding, because the combination of two or more atoms usually has a more stable electronic configuration than a single atom. The exact nature of the chemical bonds that form between atoms can vary, but it invariably involves the sharing or exchange of electrons.
We have already seen that an element consists entirely of atoms of just one kind. If the atoms participating in a chemical bond are all of the same kind, we have an elemental substance. If the atoms participating in the bond are of different kinds, we have a chemical compound. Most of the substances we encounter on a day-to-day basis are chemical compounds of one kind or another. Examples include water, salt and sugar.
Molecules
When atoms of two or more different elements combine, they form a chemical compound. Some of these chemical compounds (and indeed some elemental substances) exist in nature as molecules. Molecules are discrete building blocks made of two or more atoms. The atoms in a molecule can all belong to the same element, or they can belong to different elements.
A bond can form between two or more atoms of elemental gases like hydrogen, nitrogen, oxygen and chlorine. For example, two oxygen atoms (O + O) can bond chemically to form a single oxygen molecule (O2 ), sometimes referred to as dioxygen. A covalent double bond is formed between the two atoms to which each oxygen atom contributes two electrons (we will talk in more detail about the nature of chemical bonding elsewhere).The illustration below shows a dioxygen molecule. This image is one of several we created using the free cross-platform molecular editor Avogadro.
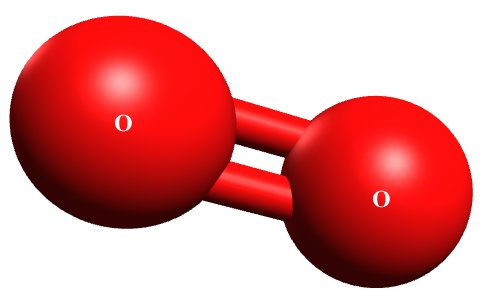
An oxygen molecule consists of two oxygen atoms
Most oxygen exists as dioxygen (O2 ), and most nitrogen exists as dinitrogen (N2 ). You are in fact surrounded by dioxygen and dinitrogen for most of the time, since the Earth's atmosphere is composed almost entirely of these two molecular gases (78% nitrogen and 21% oxygen). Other gases currently make up just one per cent of the total, and include gases like carbon dioxide (CO2 ) - a chemical compound made from carbon and oxygen. Carbon dioxide is also a molecular gas.
Water is a virtually colourless liquid that covers more than seventy percent of the Earth's surface, which is just as well considering that, without water, life as we know it would not exist. A water molecule is formed when two atoms of hydrogen bond with one atom of oxygen. The illustration below shows a water molecule.
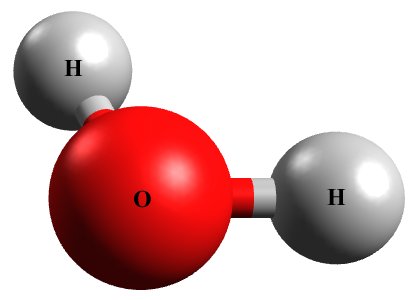
A water molecule consists of two hydrogen atoms and one oxygen atom
Molecules can be quite complex. Let's take sugar as an example. Sugar is the generic name for a whole family of carbohydrates. Carbohydrates are organic chemical compounds in which atoms of carbon, hydrogen and oxygen bond together to form molecules. The term organic refers to the fact that the compounds we are talking about are found in living organisms.
Sugar is produced in the tissues of most plants by natural processes. The kind of sugar we use to sweeten beverages like tea and coffee is called sucrose. The kind of sugar that circulates in your bloodstream (as blood sugar) is called glucose. The illustration below shows a glucose molecule.
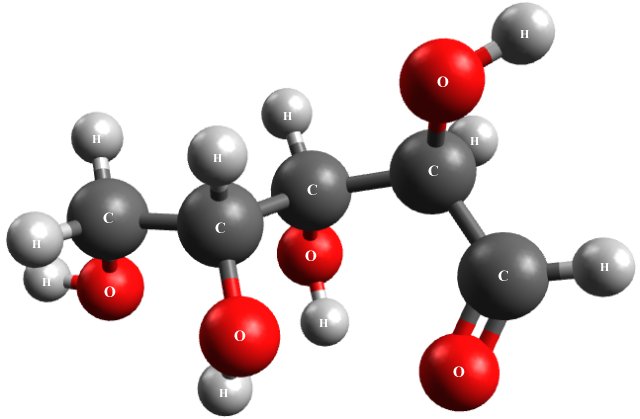
A glucose molecule is constructed from carbon, oxygen and hydrogen atoms
Note that the order in which the atoms are combined within a complex molecule, and the nature of the bonds between them, is very important. It is perfectly possible, for example, for two completely different organic compounds to be made up from exactly the same elements, with exactly the same number of atoms of each element present.
Formula units
Atoms often combine to form a continuous crystalline lattice structure rather than discrete molecules. This is the case for many solids, including most metals and metal alloys, and many chemical compounds. Nevertheless, within these crystalline structures, the atoms of the different elements involved in a compound are chemically combined in a fixed ratio.
Because crystalline substances do not form discrete molecules, we call the smallest amount of such a substance that can exist a formula unit. A specific minimum number of atoms of each consituent element must be present in order to form a single formula unit (sometimes called an elementary entity) of a particular compound.
Let's look at an example. Sodium chloride - better known as common salt - is a chemical compound found in abundance on Earth that is essential to life. It is formed when sodium atoms and chlorine atoms combine in equal numbers to create salt crystals. The resulting crystal lattice is illustrated below. Note that the smallest possible elementary entity (formula unit) of sodium chloride that can be identified as such will consist of one sodium atom and one chlorine atom.
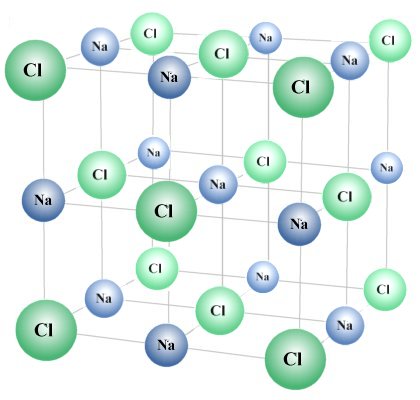
Chlorine and sodium atoms combine to form sodium chloride crystals
Chemical formulae
Chemical elements, and especially chemical compounds, sometimes have very long names. Fortunately, we don’t have to write these out in full very often. Scientists have given each chemical element a unique one or two-letter identification symbol. The letters are taken from the element’s Greek, Latin or English name. For example, the symbol for lead is Pb, from the latin word for lead, which is plumbium.
A chemical compound is identified by a formula consisting of the symbols for each element making up that compound. Numerical subscripts are used to indicate exactly how many atoms of each element make up a single molecule or formula unit of the compound. For example, a water molecule is a combination of two hydrogen (H) atoms and one oxygen (O) atom, so the formula for water is H2 O.
