Metallic Solids
In metallic solids, one or more of the electrons in each atom have sufficient energy to break away from the atom completely, resulting in the creation of positively charged metal ions that are surrounded by a "sea" of negatively charged electrons. These delocalised electrons are shared between the metal atoms, and form a kind of "glue" that holds the ions together.
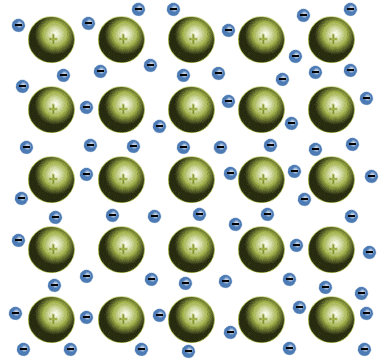
The positive metallic ions are held together by a "glue" of delocalised electrons
Although the free electrons in metallic solids hold the ions together, they are not bound to any particular atom. The ions can therefore slide past each other within this sea of electrons with relative ease, giving metallic solids (both elemental metals, and alloys of two or more elemental metals) some fairly unique properties. The precise nature of a particular metallic solid will depend on the strength of the metallic bonds, which will in turn depend on the number of valence electrons present in the metal atoms.
As a general principle, the more valence electrons there are, the stronger the metallic bonds will be. The stronger the bonds, the harder the metallic solid will be. Stronger bonds also mean higher melting points. Because the valence electrons can move around relatively freely, metallic solids are also good conductors of both heat and electricity.
A significant majority of the elements in the periodic table are metallic solids at a standard temperature and pressure (STP) of 0 °C and 1 Atm. These metallic solids can be broadly categorised as belonging to one of two groups - the transition metals (which occupy the central portion of the periodic table) and the main group metals.
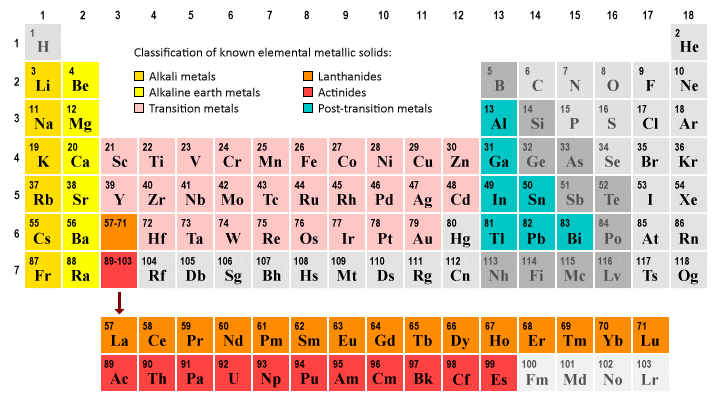
Most elements are metallic solids at standard temperature and pressure
The main group metals consist of the alkali metals and alkaline earth metals, which occupy groups one and two of the periodic table respectively, and the so-called post-transition metals, which are found in groups thirteen, fourteen and fifteen of the periodic table (some scientists consider polonium (Po) in group sixteen of the periodic table to also be a post-transition metal, but there is no overall consensus on this issue).
Two groups of elements - the lanthanides and actinides - are usually shown separately beneath the main body of the periodic table. These groups have special properties, but are sometimes considered to be part of the transition metal group of elements. We will be looking at the transition metals, lanthanides and actinides in more detail elsewhere in this section.
Most of the elements greyed out in the illustration above are either non-metals or metalloids. Mercury (Hg) is a liquid at STP. The phase and precise classification for many of the heavier elements (including all of the elements with an atomic number of 100 or greater) has still to be precisely determined at the time of writing. The actinides Fermium, Mendelevium, Nobelium and Lawrencium, for example, are all predicted to be solids at STP.
