Network Covalent Solids
A network covalent solid (or just network solid) consists of a network of atoms of the same or different elements connected to each other by covalent bonds. The network of covalent bonds extends throughout the crystalline structure. We can think of this structure as being rather like a giant molecule - except that the number of atoms is not fixed, and will depend on the size of the structure.
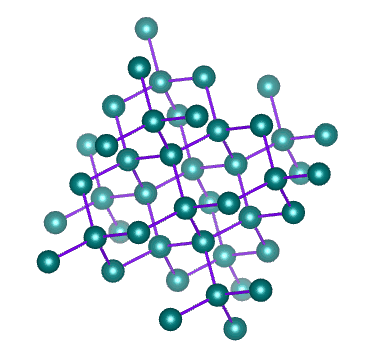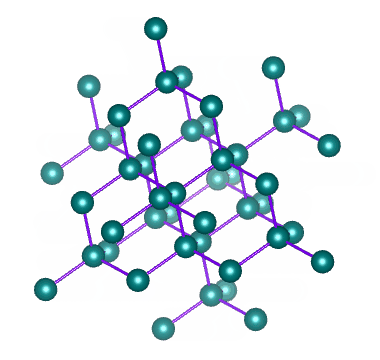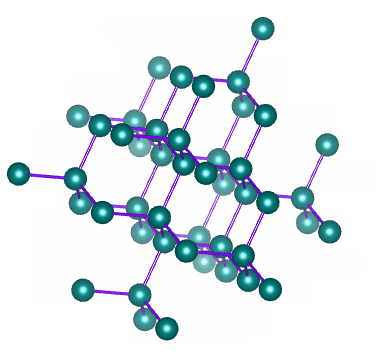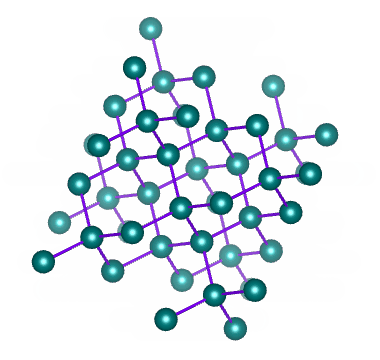
Diamond is a network of carbon atoms connected to each other by covalent bonds
The animation above was created using VESTA (Visualization for Electronic and STructural Analysis), a software package developed by Koichi Momma and Fujio Izumi that is distributed free of charge for academic, scientific, educational, and non-commercial use.
A network solid does not have discrete molecules; the smallest amount of a network solid that can be identified as such is called a formula unit. Examples of network covalent solids include diamond and graphite (both allotropes of carbon), and the chemical compounds silicon carbide and boron-carbide.
Network covalent solids tend to be hard and brittle (graphite is a notable exception, because its covalent network takes the form of a two-dimensional sheet of graphene just one atom thick), and have high melting and boiling points. Silicon carbide (also known as carborundum) is a compound of silicon and carbon. Boron carbide is a compound of boron and carbon.
Both silicon carbide and boron carbide are hard ceramic materials that have numerous applications. Silicon carbide is used in the manufacture of automotive brake and clutch parts, as an abrasive material, and as the main component in bullet proof vests and body armour. Boron carbide is probably the second hardest material after diamond, and is used to provide armour plating in armoured vehicles and military aircraft.
The hardness and high melting and boiling points of network covalent solids stems from the fact that the covalent bonds holding them together are not easily broken. Diamond is the hardest substance known to man, and has a melting point of almost four thousand degrees Celsius (4,000 °C).
Most network covalent solids are poor conductors of electricity because all of the valence electrons are involved in covalent bonds. Graphite is one of the few exceptions, as only thee of the four valence electrons in the carbon atoms are involved in covalent bonding; the fourth valence electron is delocalised, and can thus move freely between the graphene layers, acting as a current carrier.
Network covalent solids are generally insoluble, because the attraction between the solvent molecules and the covalently bonded atoms is far too weak to overcome the the strength of the covalent bonds.

