Solids - an Overview
A substance in its solid state tends to maintain a constant shape and volume because its elementary entities (atoms, molecules or ions) do not possess enough kinetic energy to allow them to overcome the forces that hold them tightly in place. At temperatures approaching absolute zero (-273.15 °C), virtually all known substances are solids. The substance with the lowest melting point is helium, with a melting point of minus two hundred and seventy-two point two degrees Celsius (-272.2 °C). Helium is the second lightest and the second most abundant substance in the observable universe, after hydrogen.
Solids share some of the characteristics of other forms of matter. All matter has mass and volume, for example. All matter is composed of elementary entities such as atoms, molecules, ions and electrons. The defining characteristics of solids - the things that make them solids, as opposed to liquids, gases or plasma - are described below.
Looking beyond these common characteristics, however, we will see that solids can exist in many different forms. They can be hard or soft, rigid or flexible. They may or may not conduct electricity or heat. They can consist of a single substance (an element or chemical compound), or may be formed from a combination of substances. They can even exist in highly complex organic structures. In fact, most of the solids which we encounter on a daily basis are either inorganic mixtures (e.g. earth, stone, concrete, bricks and mortar), or organic materials (e.g. wood, paper, cardboard, rubber, leather).
In this section, we will look at the different kinds of solids that exist in the world around us, and look at some of the many ingenious ways in which man has used those materials. We will also be looking at the way in which solid materials are classified. Solid materials are very broadly split into two main categories:
- Crystalline solids - most solids fall into this category, including all elemental metals and non-metals and all chemical compounds (in their solid state). In crystalline solids, the particles (atoms, molecules or ions) are tightly packed together in a regularly repeating three-dimensonal pattern. The resulting crystalline structure is often referred to as a crystal lattice.
- Amorphous solids - this category of solids includes materials such as rubber, polymers and gels and - perhaps somewhat surprisingly - glass. Amorphous solids are characterised by their lack of a crystalline structure - they are said to lack the "long-rang order characteristics" of a crystalline structure. This essentially means that the atoms and molecules in an amorphous solid are arranged in a more or less random fashion, as opposed to being organised into a definite crystal lattice.
As we have seen, the elementary entities (we will refer to them simply as particles from now on) that make up a solid are locked into position within a rigid structure with little space between them - usually, though not always, in a regular pattern. The particles that make up a solid cannot break free of the interatomic or intermolecular bonds that hold them in place. Nevertheless, they will possess some kinetic energy - even at temperatures approaching absolute zero - and will vibrate around their fixed positions.
The amount of space between the atoms - and there will always be some space between them, no matter how tightly packed they are - varies from one substance to another. A standard house brick, for example is much heavier than a block of wood with the same dimensions, because it has a higher density. That's partly because the particles that make up the brick are packed more closely together than the particles that make up the block of wood, but it's not the only reason.
The density of a substance is defined as the amount of mass the unit has per unit volume. While partly dependent on the spacing between individual particles, it also depends on the mass of the particles themselves. For example, a single atom of the element gold has a total of one hundred and ninety-seven nucleons (neutrons and protons) in its nucleus. This makes an atom of gold far heavier than an atom of the element carbon, whose nucleus has only twelve nucleons.
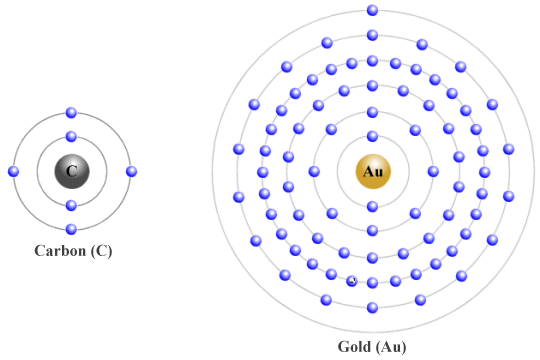
A gold atom has more than sixteen times the mass of a carbon atom
As a general rule, a substance in its solid state is significantly denser than the same substance in its liquid or gaseous state, regardless of its chemical composition, because its particles are more tightly packed when it is in the solid state than when it is a liquid or a gas. One notable exception to this is water.
When water freezes to become a solid (ice), it acquires a crystalline structure (more about that later) in which the particles are spaced further apart than they are in the liquid state. Ice is thus less dense than water. As a consequence, ice that forms in a body of water rises to the surface. A layer of ice will form on the surface of a pond in winter when the temperature drops below zero. In arctic regions, even the sea freezes - typically to a depth of several feet - covering the water beneath it. The deepest layers of water will be the last to freeze. If this were not true, life may have evolved very differently on our planet - if at all.

Sea ice in Hudson Bay, north eastern Canada
A solid tends to hold its shape; its volume will not vary significantly, and it does not possess fluidity (i.e. it cannot flow freely like a liquid or a gas). Unlike a liquid or a gas, a solid placed into a container does not adopt the shape of the container. And, because the particles are tightly packed together, a solid cannot be easily compressed.
Having said that, if we apply enough pressure to a solid, we can crush it or break it. We can use a hammer to smash a brick or a stone slab into pieces, for example. We can use tools like knives or saws to cut wood or metal. And we can use other tools to grind solid substances into a powder to make them easier to store and use. In some cases, we can transform a solid material like copper or iron by heating it until it melts, pouring the resulting liquid into a mould, and allowing it to cool again to form a solid casting with the required shape.

Metal being melted in a ladle, ready for casting
Indeed, not all materials that we regard as solids are hard, and not all solids have a rigid structure. Think about modelling clay, for example. It is soft and malleable, and can be shaped easily by hand. Most of us have at some point in our lives used modelling clay or plasticine - a clay-like material - for making models of various things.
The properties of a particular substance in its solid form will determine how we use it. Some materials, like concrete and steel, have a high tensile strength and are used for the construction of buildings and bridges. Certain metals - notably copper and tin - are good at conducting electricity, and can be stretched into long thin wires. This makes such materials an excellent choice for the manufacture of all kinds of electrical cable. Other materials, such as diamond, are extremely hard, and can be used for processes involving cutting or drilling.

Materials like concrete and steel are used in construction
Ceramic materials that retain their strength at high temperatures can be used for all kinds of things, from tableware and ceramic hobs to heat shields for spacecraft. Ceramic materials also tend to be good electrical insulators, and are used as such in many high voltage applications, including electrical power distribution and electrified railway systems.
Materials like glass and certain plastics have optical properties that make them ideal for countless applications. The glass windows in your house provide you with light during the daylight hours, allow you to enjoy the view, and protect you from adverse weather conditions. Glass is used in the manufacture of the lenses and mirrors found in optical instruments such as telescopes and microscopes. And both glass and plastic are used to manufacture the optical fibres used to transmit huge amounts of information around the globe every day at almost the speed of light.
